Material properties are “well” defined. If one goes to a reference source, the various properties of a pure material can be found. For example, the atomic number of Lithium is 3. It has an atomic weight of 6.941. Its specific gravity is 0.534, with a melting point of 180.5C, and a boiling point of 1,347C. Similarly, the atomic number of gold is 79 with an atomic weight of 196.9665. Its specific gravity is 19.32 (20C), with a melting point of 1,064 and a boiling point of 2807C.
From last month’s blog, the “bulk” material properties may not represent the properties of nanoparticles of the material. Consequently, there is a need to consider various individual isotopes of the materials that are being investigated. The chart below {Ref. #1] shows the change in melting point as the size of the gold nanoparticle diminishes. (Other examples are available in my September 14th, 2013 blog.) The melting point of a number of materials starts to decrease when the particle size is near 50nm. 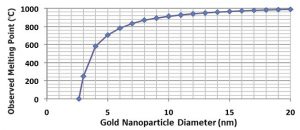 What is the effect of increasing pressure? Will it increase or decrease the melting point and/or the boiling point? The answer is not initially obvious. The answer is dependent on whether the material becomes more dense or less dense at higher temperatures. If the material requires more space as it melts, the higher pressure increases the melting point. If the material requires less space as it melts (think water), then higher pressure decreases the melting point. The boiling point typically has an inverse relation with vapor pressure of the liquid and a positive relationship with atmospheric pressure.
What is the effect of increasing pressure? Will it increase or decrease the melting point and/or the boiling point? The answer is not initially obvious. The answer is dependent on whether the material becomes more dense or less dense at higher temperatures. If the material requires more space as it melts, the higher pressure increases the melting point. If the material requires less space as it melts (think water), then higher pressure decreases the melting point. The boiling point typically has an inverse relation with vapor pressure of the liquid and a positive relationship with atmospheric pressure.
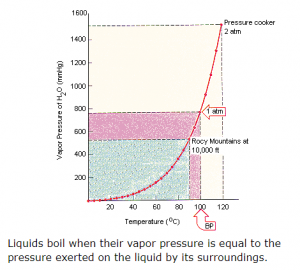 The figure above [Ref. #2] shows the relationship of the boiling point of water to pressure. (Consequently, the need for pressure cookers at higher altitudes for proper cooking temperatures.)
The figure above [Ref. #2] shows the relationship of the boiling point of water to pressure. (Consequently, the need for pressure cookers at higher altitudes for proper cooking temperatures.)
The figure above is an excellent visual representation of the impact of altitude (pressure) on the boiling point of water. Similar graphs could be done for gold or lithium. But how can one visualize the impact of the change in size at the nano realm. Does there need to be another chart for 10nm gold and another for 15nm gold, etc. Or would it be better to have a single pressure point and look at the change in melting point, which is the first figure. Would it then be necessary to have a multitude of curves at various particle sizes? Is it possible to develop a three-dimensional graph that has temperature, pressure, and size as the axes? That could be done.
What happens when the other properties of the materials need to be considered. Do the electrical properties change as the material becomes smaller? Yes. Do the magnetic properties change? One could indicate no, but that would not explain the magnetic moment of 13 atoms of silver. Does the ability of the material to interact with other materials change with size? Again, the answer is yes. Can the material change shapes? Certain ones can. The list of differences at the nano realm is very large.
How should this be handled? There is a need to define key characteristics of nanomaterials that have a strong impact on the performance of the materials. (Do we also do this for each of the predominant isotopes?) Initially this effort needs to be established at 50, 45, 40, 35, 30, 25, 20 nanometers. Below 20nm, it should be done at 2 nanometer intervals. It is a lot of work, but to fully understand and apply the nanomaterials, the information is needed. Each time new characteristics are determined, that data needs to incorporated into the available data.
The objection could be that there is no means of using all the multiple charts that would be developed. The development of an effort like this can apply the rapidly developing field of augmented reality. The ability to flip through various three-dimensional graphs to observe trends is easier if the data can be projected, rotated, and translated. Visualization is a powerful tool. We need a lot more data on nanomaterials and a means of using the data.
References:
- http://en.wikipedia.org/wiki/Melting-point_depression
- http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch14/melting.php
